
For us it is important to realize that the electron forms some kind of standing wave. This is described in detail in all textbooks on quantum mechanics.

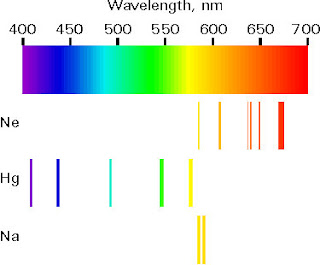
Its possible wavefunctions can be obtained as solutions of the Schrödinger equation. Its nucleus carries one unit of positive elementary charge and thus binds only one electron to it. The hydrogen atom is the simplest of all atoms. Wavefunctions are used to calculate observable quantities in particular, the probability to find the (pointlike) particle in some volume is given by the squared value of the wavefunction integrated over the volume. Thus the electrons bound by electric force to an atomic nucleus (which contains almost all of the atom's mass) must be considered to be waves. The fact that these apparently contradictory attributes are compatible in matter waves and also in light (photons) is hard to understand, but all experimental data point out that this is the case. Waves always have some spatial extension, while one may imagine the elementary, indivisible particles as being “pointlike”. While we cannot dive into mathematical details here, the basic principles shall be sketched. Quantum theory is, so to say, the mathematical formulation of particle–wave duality. Only with quantum theory atomic structure can be understood. Nevertheless, to understand how the colours which surround us come about, one needs some basic knowledge on the smallest parts of matter. If the light of the sun is spread out into different colours by a simple glass prism, the narrow absorption lines cannot be seen. Neon, which gives red colour in a gas discharge, is a colourless gas. The aurora borealis (northern light) is very rare at our latitudes, and to appreciate the colours of cosmic objects, powerful telescopes are necessary. Neon signs (or other gas discharge tubes) as used for advertising, sodium or mercury vapour lamps show atomic emission the colours of fireworks are due to it. Light emitted or absorbed by single atoms contributes only very little to the colours of our surroundings. The same file with adapted formatting can be found here. Atomic spectra The html formatting and custom instructions have been disabled on this server.


 0 kommentar(er)
0 kommentar(er)
